How Many Unpaired Electrons Are in Lithium
This storm is A. Recall from the chapter that a diamagnetic substance is identified by the lack of unpaired electrons in its shell.

Chemistry The Central Science Chapter 6 Section 8
Up to 256 cash back How many unpaired electrons do lithium have.

. Each pair of electrons is 2 electrons which means that in the case with bromine where there are 7 valence electrons there are 3 pairs and 1 left over which means there is 1 unpaired electron. And electrons equal to protons are located outside the nucleus. The only answer choice without unpaired electrons in its ground state is helium.
The ground state electron configuration of a phosphorous atom has three unpaired electrons. This atom is A. See answer 1 Lithium atoms contain one unpaired electron.
Colored according to the elements with one unpaired electron to those with five or more unpaired electrons. So there are 4 unpaired electrons. How many electrons are in Na.
The net charge of lithium atom is zero the charge of the unpaired 2s electron is obviously -mathrme. How many electrons are in the highest occupied level of an atom of aluminum. How many unpaired electrons are in the lithium atom.
The answer is C 3. Which means that Lithium atom has 1 unpaired electron. This corresponds to the 2s and 2p orbitals.
Two of the three total electrons in a lithium atom are paired in its lowest energy s orbital which can contain only two. In the lithium atom the two electrons in the 1s orbital are paired and the electron in the 2s orbital is unpaired. Desde los elementos con un electrón desapareado hasta aquellos con cinco a más electrones desapareados.
Diamagnetic How many unpaired electrons are in the titanium atom. How many unpaired electrons are in lithium. 1 Li 3 atomic number 1s22s1 so it have 1 unpaired electrons View the full answer.
For finding the number of unpaired electrons then first we have to find the atomic number of the element then write the configuration in the ground state then according to the oxidation state subtract the number of electrons. In the lithium atom the two electrons in the 1s orbital are paired and the electron in the 2s orbital is unpaired. How many unpaired electrons are in the lithium atom.
The 3d sublevel has five orbitals each of which can contain two electrons with opposite spin Pauli exclusion principle for a total of 10. MgSO4 NiSO46H2O CoCl26H2O KCrSO4212H2O CuSO45H2O NH42Cr2O7 FeSO47H2O MnCl24H2O FeCl36H2O K3MnCN6. The spin of a lithium nucleus depends on the isotope.
Indicate the charge of each species. From the periodic table we see that the atomic number of lithium is three. That is we can finally say that there are electrons equal to the atomic number in the lithium atom.
How do you find unpaired electrons. 6ceLi has the spin 1 7ceLi has spin 12. Electrons are fermions so the unpaired mathrm2s electron has the spin 12.
Orbitals are filled in order of increasing energy with no more than two electrons per orbital. Solve any question of Structure of Atom with-. Find the electron configuration to name the number of unpaired electrons in an atom of this element.
The electrons in 1s shell are paired. How many unpaired electrons are present in a lithium atom. A quantum is a _____ amount of energy.
How many paired and unpaired electrons are there in phosphorus atom. How many unpaired electrons are present in a lithium atom. For a total of 15 electrons.
The atomic number of phosphorus is 15 and the 3p sublevel has three unpaired electrons. The atom of sodium has 11 electrons 11 protons along with 12 neutrons but Na contains one less electron 11 protons along with 12 neutrons as the ion has lost 1 electron. The electron in 2s shell is unpaired.
The way in which electrons are distributed among the various orbitals is called the electron configuration. 2s 2p If n 2 then l 0 1. Lithiums electron configuration is 1s2 2s1.
1E23 unpaired electrons For there to be an unpaired electron there must be an odd number of valence electrons. For finding the number of unpaired electrons then first we have to find the atomic number of the element then write the configuration in the ground state then according to the oxidation state subtract the number of electrons from the outer shell. If n2 what types of orbitals are possible.
However when adding electrons to the orbitals within a sublevel one electron will be added to each orbital and each with the same spin Hunds rule. These two electrons would have opposite. For finding the number of unpaired electrons then first we have to find the atomic number of the element then write the configuration in the ground state then according to the oxidation state subtract the number of electrons from the outer shell.
Chemistry questions and answers. M n 3 this is the ion of manganese and the atomic number of manganese is 25. Select all that apply 1s 2s 2p 3s 3p 3d.
So it will have only 2 unpaired electron. Periodic table with unpaired electrons. Lithium-6 has an atomic mass of 6015 amu.
Theyre in the 3p sublevel. We would thus begin by placing two electrons in the 1s ground state or lowest energy orbital. Write the chemical reaction of lithium metal in a solution of silver nitrate.
Lithium-7 has an atomic mass of 7016 amu. How many unpaired electrons are present in a neon atom. Lithium atom has 3 electrons in total.
How many single electrons in N2 atom. So there are 3 unpaired electrons. There are 10 electrons present in Na.
Find the number of unpaired electrons on the central metal atom of following. N2 is the formula for a molecule of nitrogen and has no unpaired single electrons. Correct option is B As we know electronic configuration of Ni 2Ar3d 8.
Tabla periódica con electrones desapareados. Lithium is an element with atomic number 3 symbolized by the abbreviation Li. The ground state electron configuration is Ar3d742.
This element has 3 electrons. For finding the number of unpaired electrons then first we have to find the atomic number of the element then write the configuration in the ground state then according to the oxidation state subtract the number of electrons from. The electron configuration is.
In the lithium atom the two electrons in the 1s orbital are paired and the electron in the 2s orbital is unpaired.

Solved How Many Unpaired Electron Are In The Lithium Atom Chegg Com
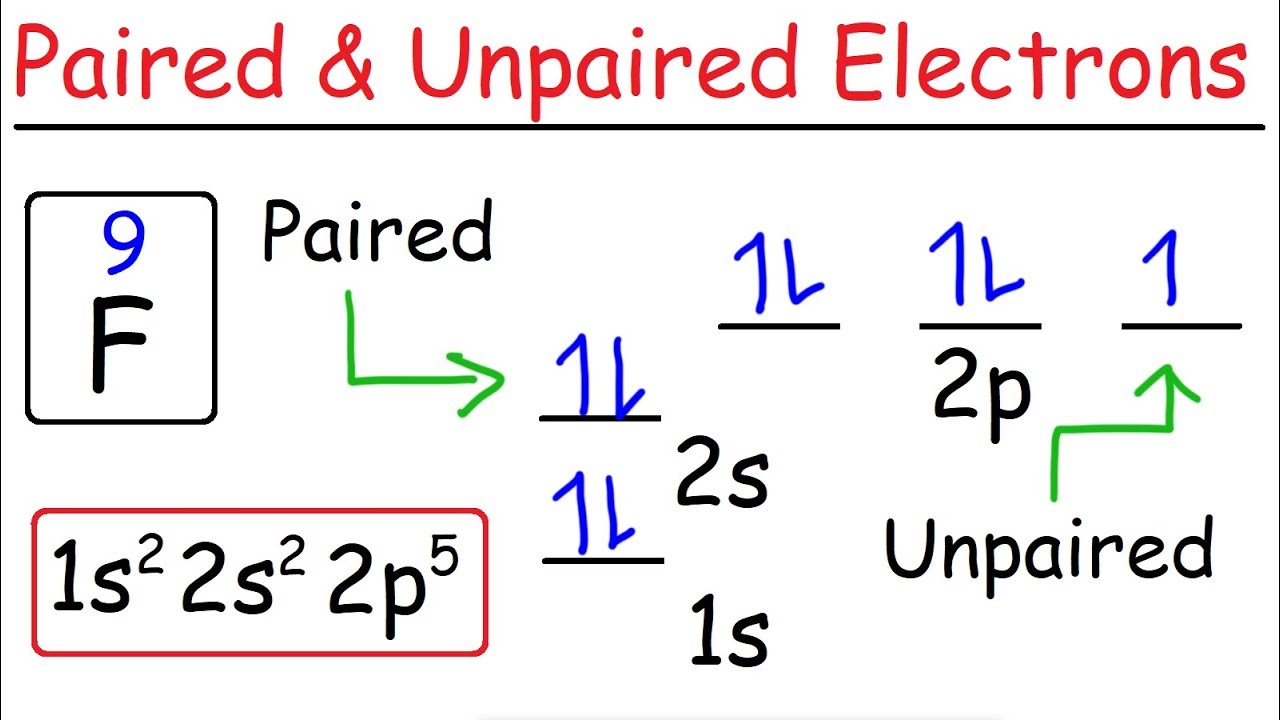
How To Determine The Number Of Paired And Unpaired Electrons Youtube

Solved How Many Unpaired Electron Are In The Lithium Atom Chegg Com
0 Response to "How Many Unpaired Electrons Are in Lithium"
Post a Comment